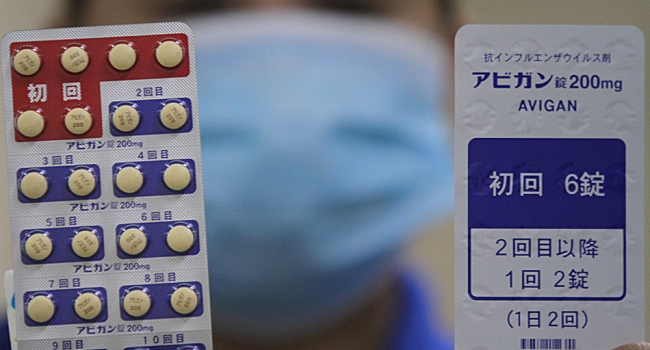
The anti-flu drug ‘Avigan tablets’ from Japan will start its trial as a COVID-19 vaccine in the Philippines on Monday, to be given to 100 patients.
According to Health Undersecretary Maria Rosario Vergeire on a report from ABS-CBN News,
The list of hospitals will be expanded for us to be able to have these 100 patients who will receive the allocated drugs coming from the Japanese government.
For 9 months, 100 patients from four hospitals in Metro Manila would be put on trial to study the efficacy of the Japanese-made anti-flu drug, she stated.
One of the candidates for the COVID-19 vaccine that is being developed worldwide is Avigan. And the other one is the Russia’s “Sputnik V” which was reportedly to be given for free.
Avigan is the brand name of the drug favipiravir. It was developed by what is now known as Fujifilm Toyama Chemical and approved for use in Japan in 2014.
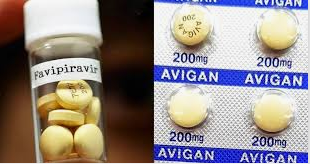
However in Japan, it is only approved for use in flu outbreaks that aren’t being effectively addressed by existing medications. It is not available on the market and can only be manufactured and distributed at the request of the Japanese government.
The first set of patients would be given prior supportive care available at the hospitals. Afterward, the second group would receive the same care and given doses of the new Japanese antiviral drug. However, Vergeire added that only patients aged 18-74 may participate in the study, as per the report.
Additionally, contraceptives are required for the trials. This was due to Avigan being teratogenic which may cause birth defects. Meanwhile, P18 million had already been allocated by the government for the clinical trials of Avigan.
Fujifilm Holdings announced last week that it expects to finish trials in Japan around September and would seek approval. By that time, Russia’s Sputnik V also hoped to finish the last phase for their clinical trials.
What can you say about this? Share it in the discussion box below.