Researchers reported on Monday that three patients whose lower bodies were completely paralyzed as a result of spinal cord injuries were able to walk, cycle, and swim using a nerve-stimulation device controlled by a touchscreen tablet.

The patients’ injuries to the thoracic spine—below the neck and above the lowest part of the back—had occurred one to nine years before they received the treatment. After neurosurgeons implanted prototypes of a nerve-stimulation device remotely controlled by artificial-intelligence software, they have been able to take their first steps within an hour.
The patients regained the ability to engage in more advanced activities—walking, cycling, and swimming in community settings outside of the clinic—over the next six months by controlling the nerve-stimulation devices themselves using a touchscreen tablet, according to the researchers.
The patients, all men aged 29, 32, and 41, were involved in motorcycle accidents.
The study was led by Grégoire Courtine and Jocelyne Bloch of the Swiss Federal Institute of Technology in Lausanne and published in the journal Nature Medicine. They assisted in the formation of Onward Medical, a future tech company based in the Netherlands that is functioning to commercialize the platform.
According to Courtine, the company plans to start a trial with 70 to 100 patients, primarily in the United States, in about a year.
So there’s no traditional treatment that allows the spinal cord to recover itself, but researchers have looked into ways to help paralyzed people recover movement using technology.

If the preliminary findings of this study are confirmed in larger studies, people immobilized by spinal cord injuries may someday be able to open a smartphone or talk to a smartwatch, choose an exercise like as “walk” or “sit,” and then deliver a signal to an implanted device that will stimulate their nerves and muscles to make the proper movements happen, according to the scientists.
Bloch stated that it is normal to initiate the movement to the brain sends a message to the spinal cord, telling it to stimulate a pool of nerve cells that in turn activate the necessary muscle.
“It’s something we don’t even think about,” Bloch said. “It comes automatically.”

Messages from the brain cannot reach the nerves following a complete spinal cord injury. Other researchers have attempted to help paralyzed patients walk by enhancing nerves in the back of the spine with broad electromagnetic currents generated by implanted devices originally designed to control chronic pain, according to Courtine. Courtine and Bloch and their colleagues redesigned the devices so that electrical signals enter the spine from the sides rather than the back. According to Courtine, this approach allows for very specific targeting and activation of spinal cord regions. They then created artificial intelligence algorithms that instruct electrodes on the device to emit signals that stimulate the individual nerves that control the trunk and leg muscles needed for various activities such as getting up.

Those who then created artificial intelligence algorithms that teach electrodes on the device to transmit signals that activate the individual nerves that control the trunk and leg muscles required for different activities such as getting up from a chair, sitting down, and walking in the proper order.
Courtine explained that the software is tailored to each patient’s anatomy.
Patients could “immediately activate their legs and step,” after the device was implanted, according to Bloch.
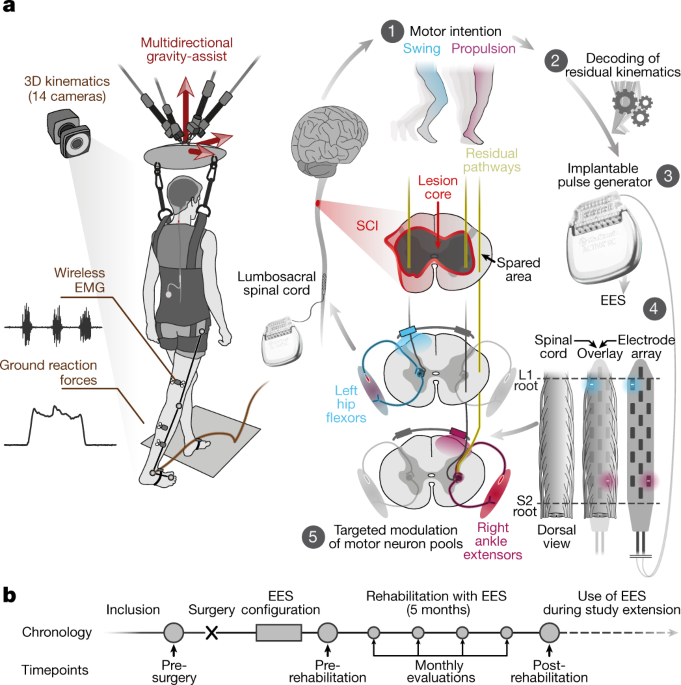
However, because their muscles were weak from disuse, they required assistance with weight-bearing and had to learn how to work with the technology, according to the researchers.
While the patients restored the capacity to perform various activities, including controlling their truck muscles, for “extensive periods,” they did not restore natural movements, according to the researchers.
“The more they train, the more they start lifting their muscles, the more fluid it becomes.” Bloch stated “.







