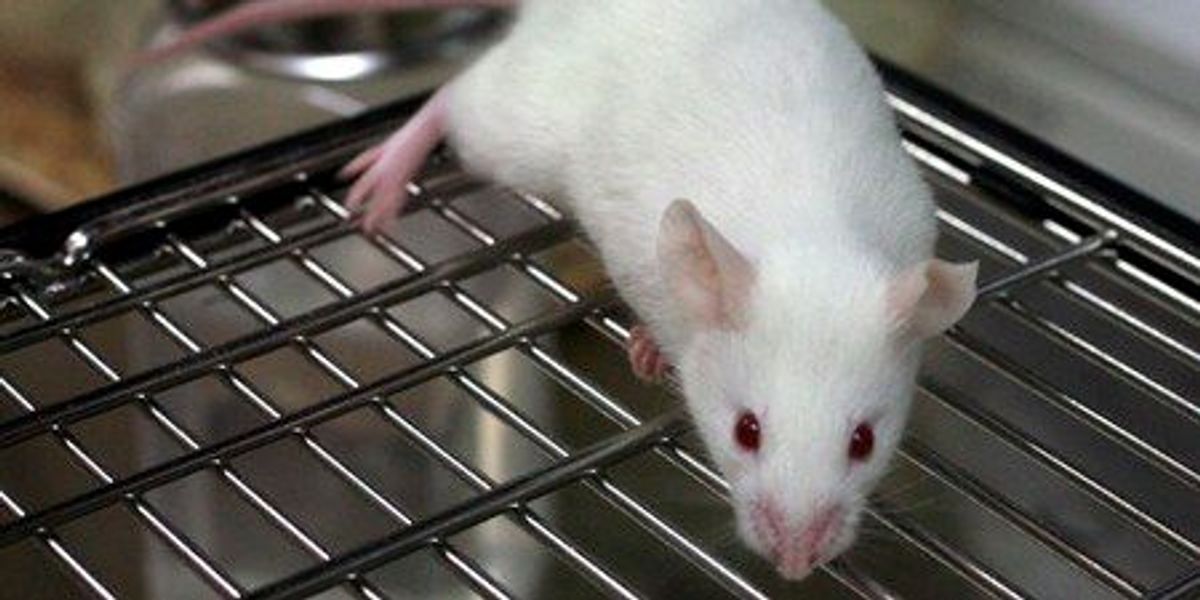
Japanese researchers have successfully cloned mice using freeze-dried cells, a technique they believe will one day aid in species conservation and overcome challenges with current biobanking methods.
The United Nations has warned that global extinctions are accelerating and that at least a million species may become extinct as a result of human-caused factors such as climate change.
Globally, facilities have sprung up to preserve samples from endangered species in the hopes of preventing their extinction through future cloning.
These samples are usually cryopreserved with liquid nitrogen or kept at extremely low temperatures, which can be expensive and vulnerable to power outages.
They also typically involve sperm and egg cells, which can be difficult or impossible to obtain from elderly or infertile animals.
Scientists at Japan’s Yamanashi University wanted to see if they could solve those problems by freeze-drying somatic cells (any cell that isn’t a sperm or egg cell) and cloning them.
They tested two types of mouse cells and discovered that, even though freeze-drying killed them and caused significant DNA damage, they could still produce cloned blastocysts—a ball of cells that develops into an embryo.
The scientists extracted stem cell lines from these and used them to create 75 cloned mice.
One of the mice lived for a year and nine months, and the researchers also successfully mated female and male cloned mice with natural-born partners, yielding normal pups.
Cloned mice produced fewer offspring than natural-born mice, and one of the stem cell lines created from male cells produced only female mouse clones.
Teruhiko Wakayama, a professor at the University of Yamanashi’s Faculty of Life and Environmental Sciences who collaborated on the study, which was published this month in the journal Nature Communications, “Improvement should not be difficult,”.
He told the AFP, “We believe that in the future we will be able to reduce abnormalities and increase the birth rate by searching for freeze-drying protectant agents and improving drying methods,”
Other disadvantages include the fact that cloning mice from cells stored in liquid nitrogen or at ultra-low temperatures has a success rate of two to five percent, whereas the freeze-dried method has a success rate of only 0.02 percent.
Wakayama, on the other hand, believes the technique is still in its early stages, comparing it to the study that yielded “Dolly,” the famous sheep clone—a single success after more than 200 attempts.
He said, “We believe the most important thing is that cloned mice have been produced from freeze-dried somatic cells, and that we have achieved a breakthrough in this field,”
While the method is unlikely to completely replace cryopreservation, Simon Clulow, senior research fellow at the University of Canberra’s Centre for Conservation Ecology and Genomics, described it as a “very exciting advance for scientists interested in biobanking threatened global biodiversity.”
“It can be difficult and costly to work up cryopreservation protocols and so alternatives, especially those that are cheaper and robust, are extremely welcome,” Clulow, who was not involved in the research, added
The freeze-dried cells were stored at minus 30 degrees Celsius, but the team has previously demonstrated that freeze-dried mouse sperm can survive at room temperature for at least a year and believes somatic cells will as well.
According to Wakayama, the technique could eventually “allow genetic resources from around the world to be stored cheaply and safely.”
Wakayama and his colleagues have spent years researching cloning and freeze-drying techniques.
One of their most recent projects involved freezing mouse sperm and sending it to the International Space Station. Even after six years in space, the cells were successfully rehydrated and produced healthy mouse pups when they returned to Earth.







