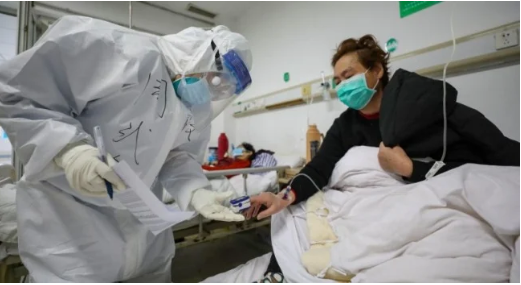
The fight against the Coronavirus had seen new developments over the past few weeks.
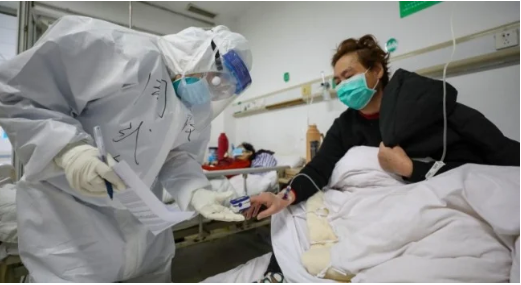
Remdesivir, is a drug that is set to be used on 761 infected patients on the NCOV-19 in Wuhan China for the critical trial.
According to the report, this medicine is also good for treating SARS and MERS Coronavirus as well as Ebola infections. In the meantime, the China National Biotec Group had introduced that virus-neutralizing antibodies were extracted from the plasma for recovered patients from COVID-19.
Furthermore, The experiments had shown the antibodies were found to be effective in fighting with the new coronavirus and as what the report from SCMP said that the company had already prepared for the medical treatment.
This is the strict blood biological safety testing, virus inactivation, and antiviral activity testing. Moreover, the plasma extracted was used to treat 11 patients that are in critical condition and had given a great result.
There are 3 critically ill patients was the first one to receive the experimental treatment in the first phase. And now the plasma is being used to treat more than 10 critical patients.
In the report, the test shows that the following 12-24 hour period of treatment is the main inflammatory signs in the lab decreased significantly.
The plasma product to treat the novel coronavirus is made from plasma loaded with antibodies donated by recovered patients. It went through virus inactivation and was tested against virus-neutralizing antibodies and multiple pathogenic microorganisms.
In addition to that, the proportions of lymphocytes have also increased. And the key indicators like blood oxygen saturation and viral load have improvements. Clinical signs and symptoms had also seen significant improvements.
What are your thoughts and comments about these improvements? Share it in the discussion box below.